Uranium is a naturally occurring radioactive element, which has the atomic number of 92 and corresponds to the chemical symbol U in the periodic table. It belongs to a special group of elements called “actinides” — elements that were discovered relatively late in history. Like all other actinides, uranium is “radioactive” – it decays over time and releases energy in the process. Its special properties make uranium the main source of fuel for nuclear reactors — a chicken-egg sized amount of uranium fuel can provide as much electricity as 88 tonnes of coal.
Uranium is among the more common elements in the earth’s crust — about 500 times more common than gold. Although it seems a very rare element, small amounts of uranium are present everywhere — in rock, soil, water, and even our bodies. There are also large amounts of highly diluted uranium in the ocean — approximately four billion tonnes.
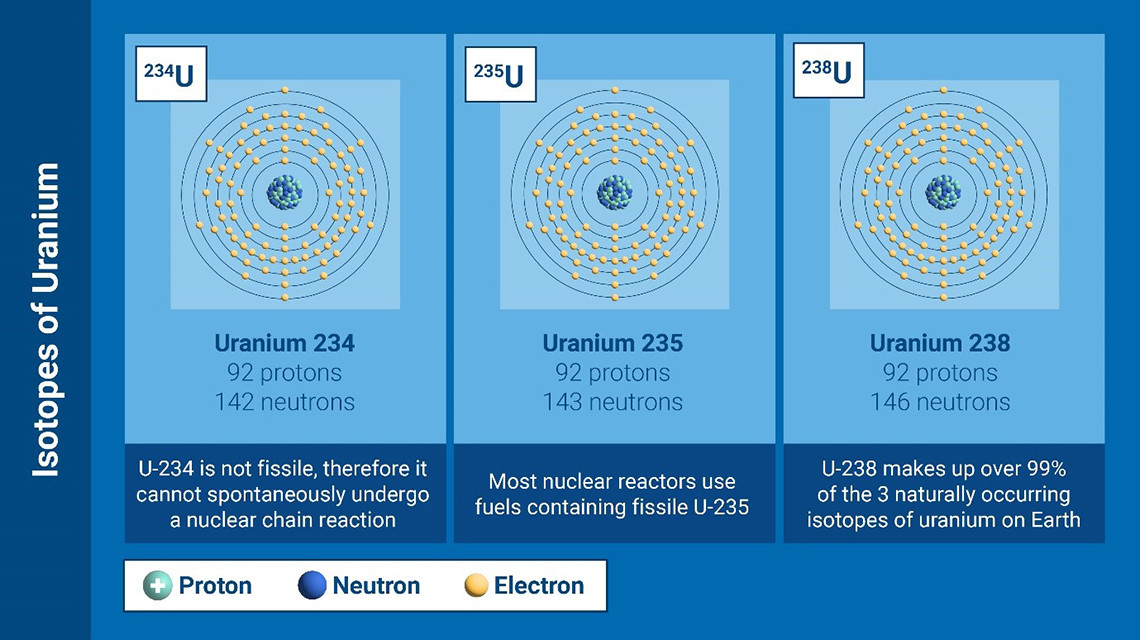
Just like any other element, uranium comes in several variations that differ in mass and physical properties but share the same chemical properties. Those are called isotopes.
This article was first published on 16 August 2023.