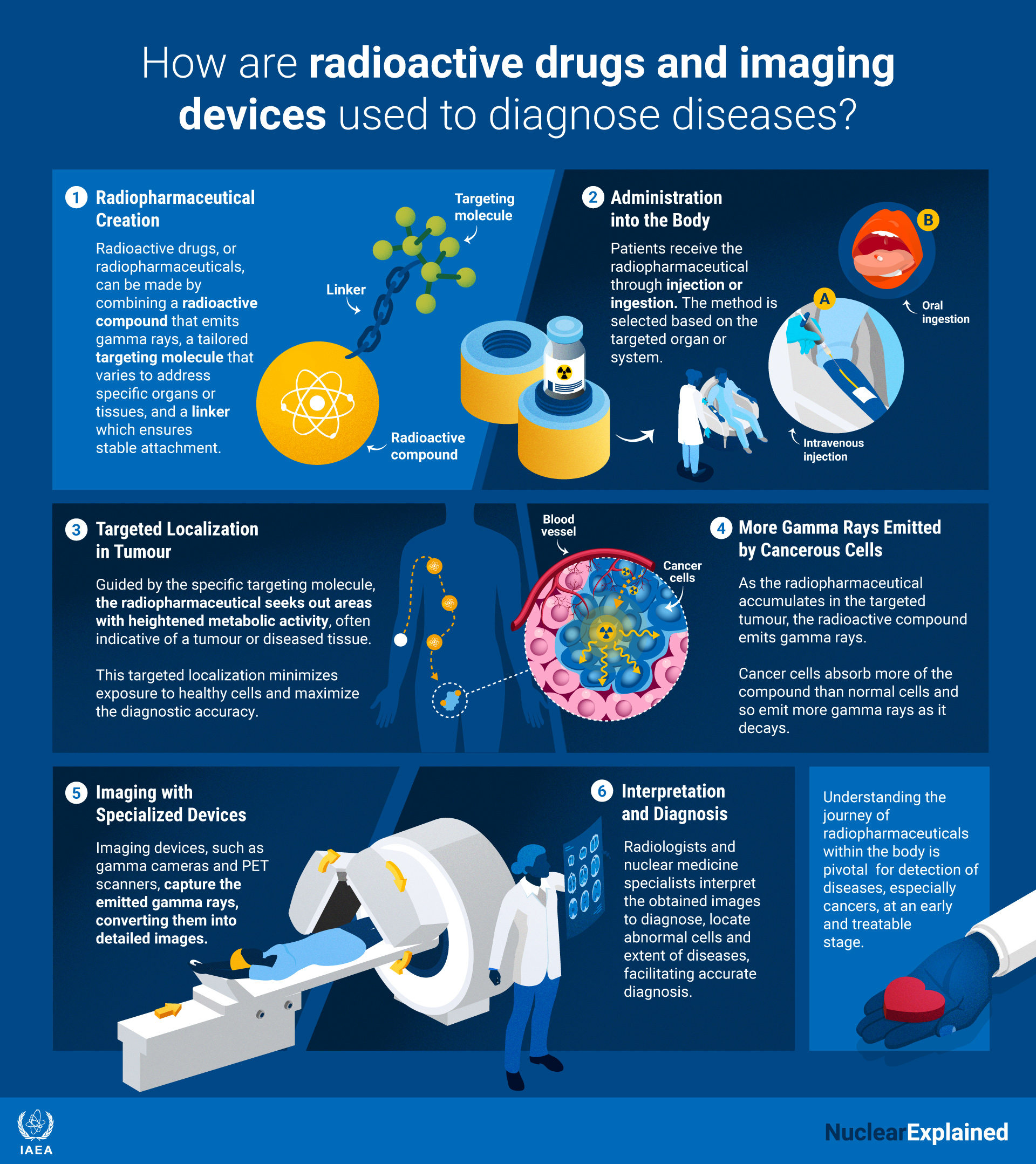
Radiopharmaceuticals are drugs that contain, among other ingredients, radioactive forms of chemical elements called radioisotopes. Depending on the type of radiation that those radioisotopes produce, they can be used to diagnose or treat several medical conditions. Their applications range from imaging of many different organs, such as brain, heart, kidney and bone, to the treatment of cancer and hyperthyroidism.
What are Radiopharmaceuticals?
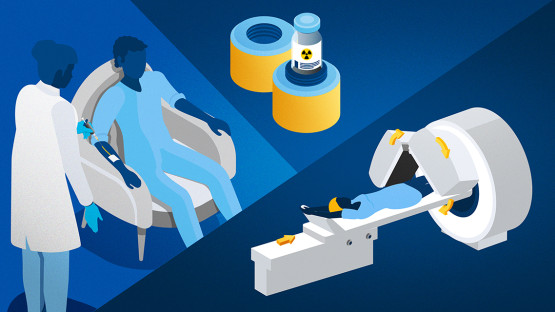
Radiopharmaceuticals are used in nuclear medicine worldwide. (Image: A. Vargas/IAEA)
Technetium-99m is the most widely used radioisotope in diagnostic nuclear medicine. Over 10,000 hospitals worldwide use it to detect cancer, cardiovascular and other chronic diseases, so that they can be treated.
Radiopharmaceuticals are given to patients by injection, or by mouth, and can be monitored and analysed with external medical devices and tests. Specialized safety protocols are in place in most countries to protect patients and health professionals from any side-effects of these drugs.
In addition to containing radioactive atoms, radiopharmaceuticals contain molecules that are designed to travel inside the body of the patient until they reach their target tissue or organ. For instance, certain radiopharmaceuticals are “sugar-like” – their radioactive atoms are part of a substance that is very similar to sugar, a glucose analogue. Since tumours consume more glucose than other parts of the body, the sugar-similar drug travels inside the body of the patient and gets absorbed to a high degree by the tumour cells that will “eat the sugar” and thereby make the tumour visible.
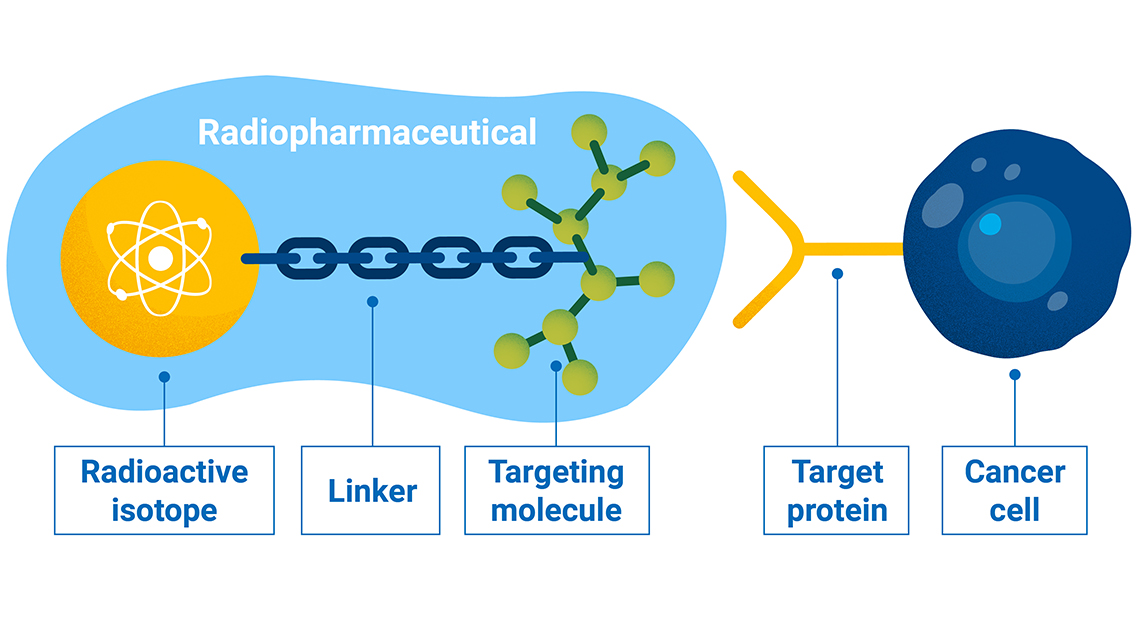
Radioactive drugs, or radiopharmaceuticals, can be made by combining a radioactive compound that emits radiation , a tailored targeting molecule that varies to address specific organs or tissues, and a linker which ensures stable attachment. This radiation could be gamma photons for diagnostic use or particles, alpha or beta, for therapy. (Infographic: A.Vargas/IAEA).
Diagnostic radiopharmaceuticals emit radiation called “(gamma) photons” which can penetrate the body and be detected by an external camera to produce images. (Graphic: A. Vargas/IAEA)
Unlike X ray examinations that can be performed at “any time”, examinations with radiopharmaceuticals often require a waiting period from when the drug is administered until it has reached the target tissue. A diagnostic radiopharmaceutical emits radiation called “(gamma) photons”. Like X rays, this special form of light can penetrate the body and detected by an external “camera” that will produce a “picture” which can be used to, for instance, see a tumour or assess the functioning of the lungs.
Radiopharmaceuticals are a key component of nuclear medicine, and are crucial to fighting cancer and several other medical conditions. Their use, however, requires extensive personnel training on patient safety and equipment handling. The IAEA has supported the establishment of a new PET Centre in Skopje, through technical advice, training of professional staff and the donation of equipment.
Following the administration of a radiopharmaceutical to a patient, health professionals typically use one of the following devices for medical imaging purposes:
- Gamma cameras: these devices detect gamma photons to produce “pictures” of the target tissue or organ.
- Single photon emission computed tomography (SPECT) scans: these devices can be used to generate 3D images because some of their parts move around the patient’s body to take “pictures” of gamma photons from all directions. These are combined to reconstruct the 3D structure of the target tissue or organ.
- Positron emission tomography (PET) scans: these devices work by recording the pairs of photons that are created each time an emitted “positron” -reacts to an “electron”. This can be used to generate high quality 3D images without the need of rotating parts.
How are radiopharmaceuticals used to treat diseases?

Iodine-131 is widely used to treat medical conditions, such as hyperthyroidism. (Photo: Adobe stock - Graphic: A.Vargas/IAEA )
Therapeutic radiopharmaceuticals contain, among other ingredients, radioactive atoms that release high-energy types of radiation -such as alpha or beta particles with short range in tissue - which destroy or weaken unwanted cells or tissues, such as tumours or overactive thyroid cells.
Diagnostic and treatment processes share similarities, however treatment with radiopharmaceuticals focuses on delivering targeted radiation to specific cells, omitting the imaging step central to diagnostic use.
While the radiopharmaceutical is designed to make it travel inside the body without harming healthy tissues, the patient may need to undergo additional tests to monitor potential side effects of this kind of therapy, which is normally considered safe and well-tolerated.
What is the role of the IAEA?
- Through its technical cooperation programme, the IAEA supports countries with technical advice, training, and equipment related to the production, the handling, and the use of radiopharmaceuticals.
- The IAEA provides practical guidance on topics related to radiopharmaceuticals and overviews the current trends in the field through technical and scientific publications, such as the IAEA Radioisotopes and Radiopharmaceuticals Series.
- The IAEA leads coordinated research projects in various nuclear fields, including nuclear medicine and the use of radiopharmaceuticals.
- The IAEA publishes Safety Standards on the use of ionizing radiation in the field of nuclear medicine.
- Through its radiation protection of patients (RPOP) portal, the IAEA provides answers to the frequently asked questions from patients and health professionals on medical procedures that involve the use of radiation, including nuclear medicine, and diagnostic and therapeutic radiopharmaceuticals.
This article was first published on 2 February 2024.