Managing water is like managing money in your bank account: you need to know exactly how much will be coming in, how much you can take out, and what could cause that to change. A miscalculation could have serious, potentially long-lasting consequences. In the world of water, this could mean water shortages or contaminated, unusable water resources.
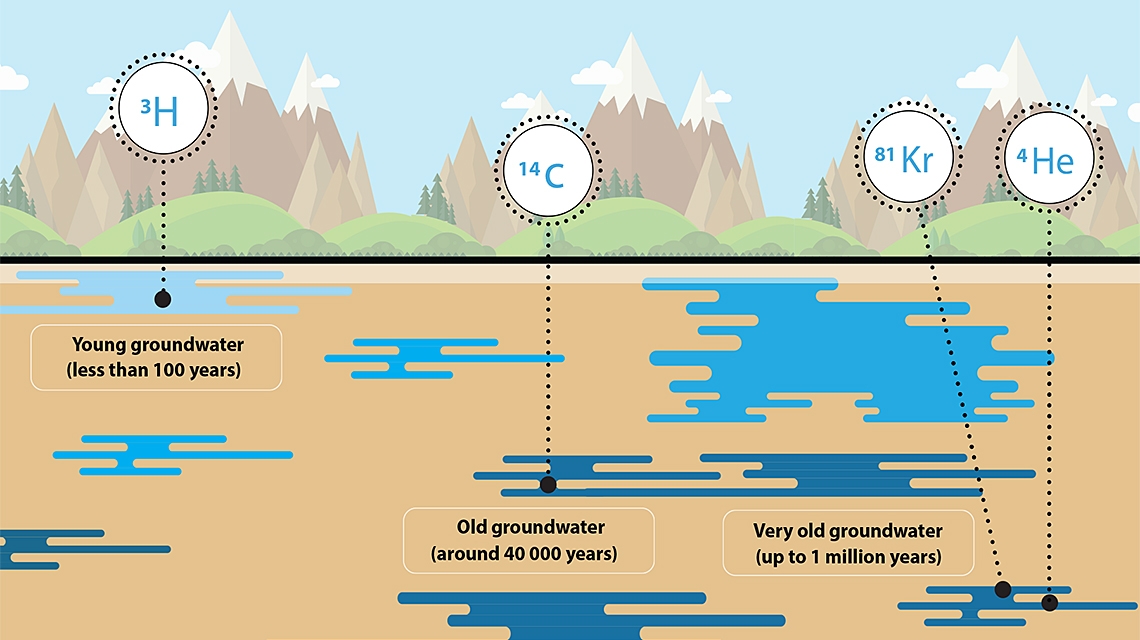
To set up a reliable water budget, one of the key factors is knowing the exact age of water. For young water, which is more likely to be affected by current climate conditions and contamination, scientists use the tritium/helium-3 technique. With this and other techniques, scientists from 23 countries are working with the IAEA to collect data about water resources.
“The age of water tells you where it most likely came from, how quickly it is replenished, and how likely it is to be contaminated,” said Hamid Marah, Scientific Director at Morocco’s National Centre for Nuclear Energy, Sciences and Technology (CNESTEN). “With the tritium/helium-3 technique, we can say if water is 1, 5 or 25 years old instead of just saying it’s young, old or both.”
The age of water can range from a few months to millions of years. If water is one year old, for example, this means it will take one year for it to be replenished and is much more likely to be affected by current climate conditions and contaminants. If water is 50 000 years old, it will take 50 000 years to be replenished and is less likely to be contaminated or affected by changes in the current climate.
Nearly all of the world’s available fresh water supplies are found in aquifers, which are the porous layers of permeable rock under the earth’s surface. The water they contain is called groundwater. As groundwater is recharged, or replenished, it eventually flows into the sea or out onto the earth’s surface naturally as rivers, springs and lakes.
“The growing demand for groundwater, combined with the impact of agriculture, climate change and human activity makes sustainability even more important,” Marah said. “By extracting too much water from an aquifer, the level of water goes down and this can be catastrophic. We are not talking about 10 to 20 years from now: its impact lasts for generations.”
The tritium/helium-3 technique is one of the most commonly used techniques for studying young water, which is water under 60 years old (see The Science box). The data collected from these studies can help decision makers develop more targeted and sustainable water resource management strategies and policies.
“Using nuclear techniques for water resource studies is breaking paradigms and changing our classical understanding of the key drivers controlling hydrological processes,” said Ricardo Sánchez-Murillo, Isotope Hydrologist and Associate Professor at the National University of Costa Rica. “In Costa Rica, for example, results from using isotopic techniques are making their way into water management plans and decision making, helping the country achieve United Nations Sustainable Development Goal 6 on water by 2030.”