CRP at a Glance
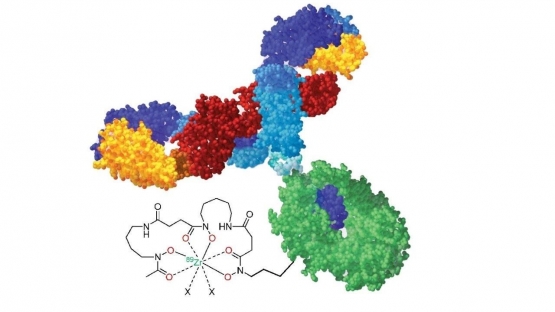
This Coordinated Research Project (CRP) will identify important technical issues related to the production of Zirconium-89 (Zr-89) and the development of Zr-89 radiopharmaceuticals. Zr-89 is a positron-emitting radionuclide with a half-life of 78.41 hours - much longer than other light elemental positron-emitting radionuclides such as Flourine-18 (F-18), Carbon-11 (C-11), Oxygen-15 (O-15) that are used routinely for molecular imaging. Zr-89 labelled molecules have opened possibilities of positron emission tomography (PET) imaging and visualization of in-vivo processes with slow pharmacokinetics, for example localization of monoclonal antibodies at cellular targets.
This CRP will address technical aspects of targets used for Zr-89 production, develop protocols for production and quality control of Zr-89 isotopes, and establish procedures for labelling and evaluating the most promising Zr-89 radiopharmaceuticals for PET imaging.
Background
Clinical significance of [18F] Fluorodeoxyglucose diagnostic imaging in cardiology, neurology and oncology has resulted in an increased number of PET imaging systems and medical cyclotrons across the globe over the last two decades. Developments in basic sciences and technology have led to the availability of new biomarkers and different biovectors, prompting the search for new PET radiopharmaceuticals with longer half-life radionuclides that match the slow pharmacokinetics of emerging biovectors such as monoclonal antibodies, cell tracking agents, nucleotides, and nanoparticle systems. The current chart of nuclides provides only a limited number of metal-element positron emitters with a sufficiently long half-life for imaging of day-long localization processes. Among these, Zr-89 stands out as a choice radionuclide mainly due to its physical decay characteristics of 78.41 hours (3.3 days) half-life and a relatively low energy positron branch (395 keV mean energy, 22.7 % intensity). The relatively long half-life of Zr-89 allows more room for regional, national and international distribution.
The availability of high specific activity Zr-89 radioisotope as a labelling precursor and standardized radiolabelling and evaluation procedures are essential for widespread utilisation of Zr-89-based products.
CRP Overall Objective
This CRP aims to formulate guidelines to enhance and strengthen the expertise and capability of Member States in the production (targetry, separation) and quality control of Zr-89 radioisotope as well as Zr-89 radiopharmaceuticals covering all aspects of development for clinical diagnostic imaging.
Specific Objectives
- Establishing guidelines for the production of Zr-89 radioisotope using medical cyclotrons through the 89Y (p,n) 89Zr reaction and the optimization of available methods (solid and liquid targets); available separation and purification methods; quality control protocols of Zr-89 radioisotope.
- Producing guidelines for the preparation, evaluation and quality control of Zr-89 labelled molecules with focus on developing Zr-89 radiopharmaceuticals including:
- biomolecule conjugation (for example, monoclonal antibodies with suitable bifunctional chelating agent);
- radiolabeling, characterization and stability studies (for example, Zr89 labelled monoclonal antibodies, cell labelling agents);
- quality control: invitro specificity evaluation (such as immunoreactivity, receptor binding, cell kinetic studies); preclinical studies in laboratory animal models.
Expected Research Outcome
The CRP aims to enhance the capabilities of Member States for the production and quality control of Zr-89 using available medical cyclotrons worldwide for developing Zr-89 radioimmunopharmaceuticals for ultimate use in early detection, screening, and follow up of human malignant diseases such as breast and intestinal cancers using PET. The CRP outcome document will contain guidelines for the production and quality control of Zr-89 radioisotope and radiopharmaceuticals using medical cyclotrons through the 89Y (p,n) 89Zr reaction, developing new generation ImmunoPET agents covering all aspects and protocols needed for radioisotope and radioimmunoconjugates.
How to join the CRP?
Please submit your Proposal for Research Contract or Agreement by email to the IAEA’s Research Contracts Administration Section, using the appropriate template on the CRA website. Applications should be submitted by 28 February 2019.
For further information related to this CRP, potential applicants should use the contact form under the CRP page.